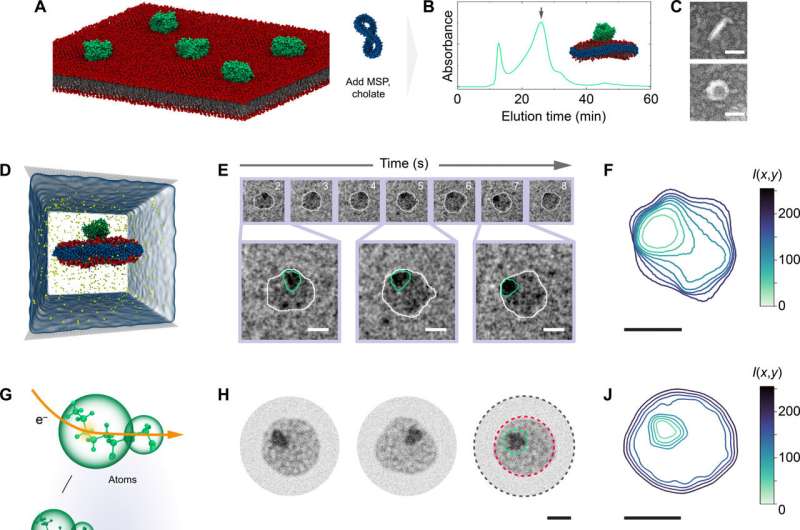
In a first demonstration of “electron videography,” researchers have captured a microscopic moving picture of the delicate dance between proteins and lipids found in cell membranes. The technique can be used to study the dynamics of other biomolecules, breaking free of constraints that have limited microscopy to still images of fixed molecules, say University of Illinois Urbana-Champaign researchers and collaborators at the Georgia Institute of Technology.
“We are going beyond taking single snapshots, which gives structure but not dynamics, to continually recording the molecules in water, their native state,” said study leader Qian Chen, an Illinois professor of materials science and engineering. “We can really see how proteins change their configuration and, in this case, how the whole protein-lipid self-assembled structure fluctuates over time.”
The researchers reported their technique and findings in the journal Science Advances.
Electron microscopy techniques image at the molecular or atomic scale, yielding detailed, nanometer-scale pictures. However, they often rely on samples that have been frozen or fixed in place, leaving scientists to try to infer how molecules move and interact—like trying to map the choreography of a dance sequence from a single frame of film.
“This is the first time we are looking at a protein on an individual scale and haven’t frozen it or tagged it,” said Georgia Tech professor Aditi Das, a corresponding author of the study. “Usually, we have to crystalize or freeze a protein, which poses challenges in capturing high-resolution images of flexible proteins. Alternately, some techniques use a molecular tag that we track, rather than watching the protein itself. In this study we are seeing the protein as it is, behaving how it does in a liquid environment, and seeing how lipids and proteins interact with each other.”
The researchers achieved videography by combining a novel water-based transmission electron microscopy method with detailed, atom-level computational modeling. The water-based technique involves encapsulating nanometer-scale droplets in graphene so they can withstand the vacuum in which the microscope operates. Comparing the resulting video data to molecular models, which show how things should move based on the laws of physics, helps the researchers not only interpret but also validate their experimental data.
“Currently, this is really the only experimental way to film this kind of motion over time,” said John W. Smith, the first author of the paper, who completed the work while a graduate student at Illinois. “Life is in liquid, and it’s in motion. We’re trying to get to the finest details of that connection in an experimental way.”
For the new study—the first published demonstration of the electron videography technique—the researchers examined nanoscale discs of lipid membranes and how they interacted with proteins normally found on the surface of or embedded in cell membranes.
“Membrane proteins are at the interface between cells and between the inside and outside of the cell, controlling what goes in and out,” Smith said. “They are overwhelmingly targets for medicine; they are involved in all kinds of processes like how our muscles contract, how our brains work, immune recognition; and they hold cells and tissues together. And all the complexity of how a membrane protein works comes from not only its own structure, but also how it’s experiencing the lipids around it.”
Electron videography allowed the researchers to see not only how the whole lipid-protein assembly moved, but also the dynamics of each component. The researchers found there were distinct regions within the nanodisc, and both more fluctuation and more stability than expected.
While it’s often assumed that the influence of a membrane protein’s movement is limited to the lipid molecules directly surrounding it, the researchers saw more dramatic fluctuations over a larger range, Smith said. The fluctuations took on a finger-like shape, like slime splatted on a wall. Yet, even after such dramatic motion, the nanodisc would return to its normal configuration.
“The fact that we saw those domains, and we saw it recover from those processes, suggests that interactions between the protein and the membrane actually have a greater range than most commonly thought,” Smith said.
The researchers plan to use their electron videography technique to study other types of membrane proteins and other classes of molecules and nanomaterials.
“We could study ion channels that open and close to regulate flow and cell-to-cell interactions using this platform,” Chen said.
Qian Chen also is affiliated with the department of chemistry, the Beckman Institute for Advanced Science and Technology, the Carle Illinois College of Medicine and the Materials Research Laboratory at Illinois.
More information:
John W. Smith et al, Electron videography of a lipid–protein tango, Science Advances (2024). DOI: 10.1126/sciadv.adk0217
Provided by
University of Illinois at Urbana-Champaign
Citation:
Electron videography captures moving dance between proteins and lipids (2024, April 22)
retrieved 23 April 2024
from https://phys.org/news/2024-04-electron-videography-captures-proteins-lipids.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
